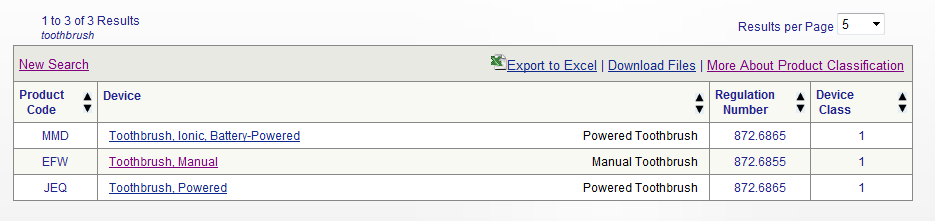
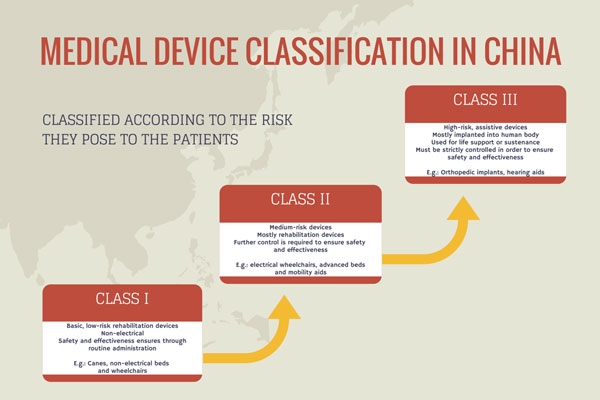
Legal Manufacturer Definition Medical Devices
This economic operator the own brand labeller meets the definition of manufacturer as set out in the medical devices directives.
Legal manufacturer definition medical devices. Own brand labeller or private labeller are therefore considered as the legal manufacturer. A natural or legal person who manufactures or fully refurbishes a device or has a device designed manufactured or fully refurbished and markets that device under its name or trademark. Legal claimed manufacturer by european directive regulations the manufacturer is responsible for the design manufacturing packaging and sale of a product placed on the european market under his own name whether he is actually fulfilling those tasks or subcontracting them. A manner that meets this definition it will be regulated as.
The pivotal provision that qualifies a medical device manufacturer as such is the characteristic of placing the medical device on the market under his own name the manufacturer clearly is responsible for the medical device manufacturing operations though the functions described can be variously delegated or subcontracted. With the publication of europe s new medical device regulation eu 2017 745 and in vitro diagnostic regulation eu 2017 746 some companies that contract manufacture or relabel devices are wondering if they will be considered the legal manufacturer of the device they sell much of the confusion around this issue stems from the assumption that there can be only one legal manufacturer. Definition of a medical device. How to comply with the legal requirements.
Manufacturer makes by chemical physical biological or other procedures any article that meets the definition of device in section 201 h of the federal food drug and cosmetic fd c act. If you are manufacturing a medical device you must follow the specific directive for your type of. For the purposes of this regulation the following definitions apply.