Lithium Hydroxide State At Room Temperature
Lioh hbr libr h 2 o uses.
Lithium hydroxide state at room temperature. It is the least dense of the solid elements and is the lightest of all the metals. It will even work well at temperatures as low as 60 o c and has been used for vehicles in the antarctic. Lithium carbonate li 2 co 3 is used as a drug to treat manic depression disorder. It has the highest specific heat capacity of any solid element.
Lithium stearate made by reacting stearic acid with lithium hydroxide is an all purpose high temperature grease and most greases contain it. Lithium hydroxide and hydrobromic acid aqueous solution of hydrogen bromide will precipitate lithium bromide in the presence of water. Herein a facile and efficient method was developed to selectively transform glucose into formic acid at room temperature. It is soluble in water and slightly soluble in ethanol and is available commercially in anhydrous form and as the monohydrate lioh.
It turns into a liquid at 453 69 k and boils at 1615 k. While lithium hydroxide is a strong base it is the weakest known alkali metal hydroxide. Lithium reacts with water but not as violently as sodium. It needs to be stored in mineral oil as it will react with air or water.
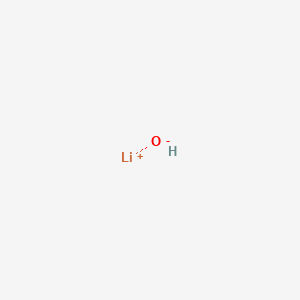
At room temperature lithium is a soft metal that is silvery white in color. It is a white hygroscopic crystalline material. After parameter optimization formic acid was obtained in an unprecedented 91 3 yield with a reaction time of 8 h in lithium hydroxide aqueous solution with hydrogen peroxide as the oxi international symposium on green chemistry 2019. Lithium hydroxide is generated by the reaction of lithium metal or lih with h 2 o and the stable chemical form at room temperature is nondeliquescent monohydrate lioh.
It loses crystalline water to form anhydride lioh almost over 423 k 150 c by heating and then it melts at 735 k 462 c which is higher than the melting temperature of naoh or koh. At atmospheric pressure and room temperature lioh crystallizes in a tetragonal structure of p4 nmm symmetry with two formula units per unit cell 6 32 33 the structure com prises layers of square lattices of lithium atoms with each square capped by a hydroxide ion alternating above and be low the lithium layer see figure 1. Lithium stearate lic 18 h 35 o 2 is used as a general purpose and high temperature lubricant. Lithium is a solid at room temperature.
Lithium hydroxide lioh is used to remove carbon dioxide from the atmosphere of spacecraft. Lithium is very reactive and flammable. A 50 60 aqueous solution of lithium bromide is used in air conditioning systems as desiccant.