Medical Device Clinical Trials In Australia

Clinical trial sponsors must be aware of the requirements to import export manufacture and supply therapeutic goods in australia.
Medical device clinical trials in australia. How clinical trials test new treatments to find better ways to prevent detect or treat disease. 2 research australia and roy morgan australia speaks research australia polling 2017 2017 at 15. The type of therapeutic good and therefore regulated as a medicine medical device or biological. Iso 14155 2011 clinical investigation of medical devices for human subjects good clinical practice 2011.
In australia however all medical devices regardless of their class must comply with the australian regulatory guidelines for medical devices argmd which includes requirements for device design and manufacturing benefits that outweigh the risks minimisation of. We regulate the use of therapeutic goods supplied in clinical trials in australia under the therapeutic goods legislation. Clinical evidence is not only required when a medical device is first included on the artg but for the entire period it remains on the register. Clinical trials help us solve really big problems and have an incredible impact on our patient s future.
Skip to content skip to main navigation skip to local. Labelling and packaging is part of the australian system of regulating medicines and medical devices. Information for health. Clinical trials posted on january 21 2016 may 4 2017 author hamish sharp categories news tags clinical trials cures devices disease drugs featured health and medical research medical research patients research australia therapies.
1 therapeutic goods administration clinical evidence guidelines. What is a clinical trial. The tables below provide a summary and comparison of the phases and stages of clinical trials involving the use of therapeutic goods. Class i devices are such low risk that they don t require clinical trials.
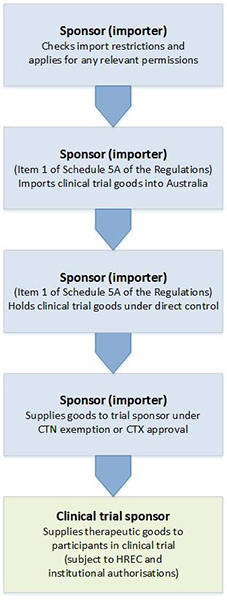
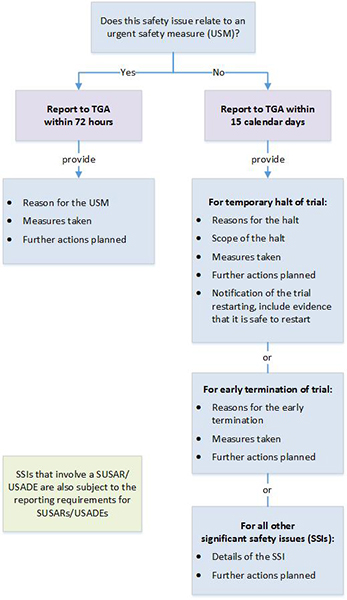
If the product is considered unapproved and therefore a ctn or ctx submission is required. Clinical trials conducted in australia are subject to various regulatory controls to ensure the safety of participants. The trial sponsor will need to determine the following for each product used in the clinical trial. Search for an australian clinical trial site.
Medical devices 24 february 2017. Clinical trials for medical devices. Clinical trials of medicines and biologicals typically proceed through phases of development whereas clinical trials of medical devices are more appropriately represented by stages. Medical devices supplied in australia must also be included on the australian register of therapeutic goods artg unless exempt or excluded 1.