Medical Device Software Testing Tools

Develop and test advanced algorithms and entire systems before implementation.
Medical device software testing tools. Matlab and simulink enable engineers to speed up medical device software and hardware development by efficiently integrating and automating the various phases of design implementation and verification. Failure proof your medical device with expert testing. Iec 62304 is applicable to all software for medical devices and applications and covers the processes and activities around the production of embedded and free standing software. Learn how the right toolset makes medical device compliance easy.
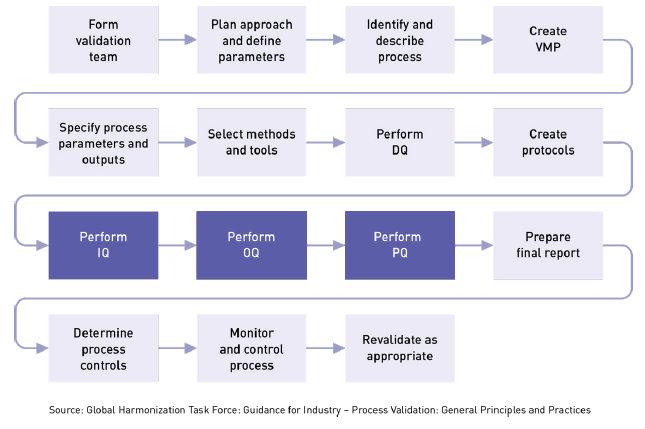
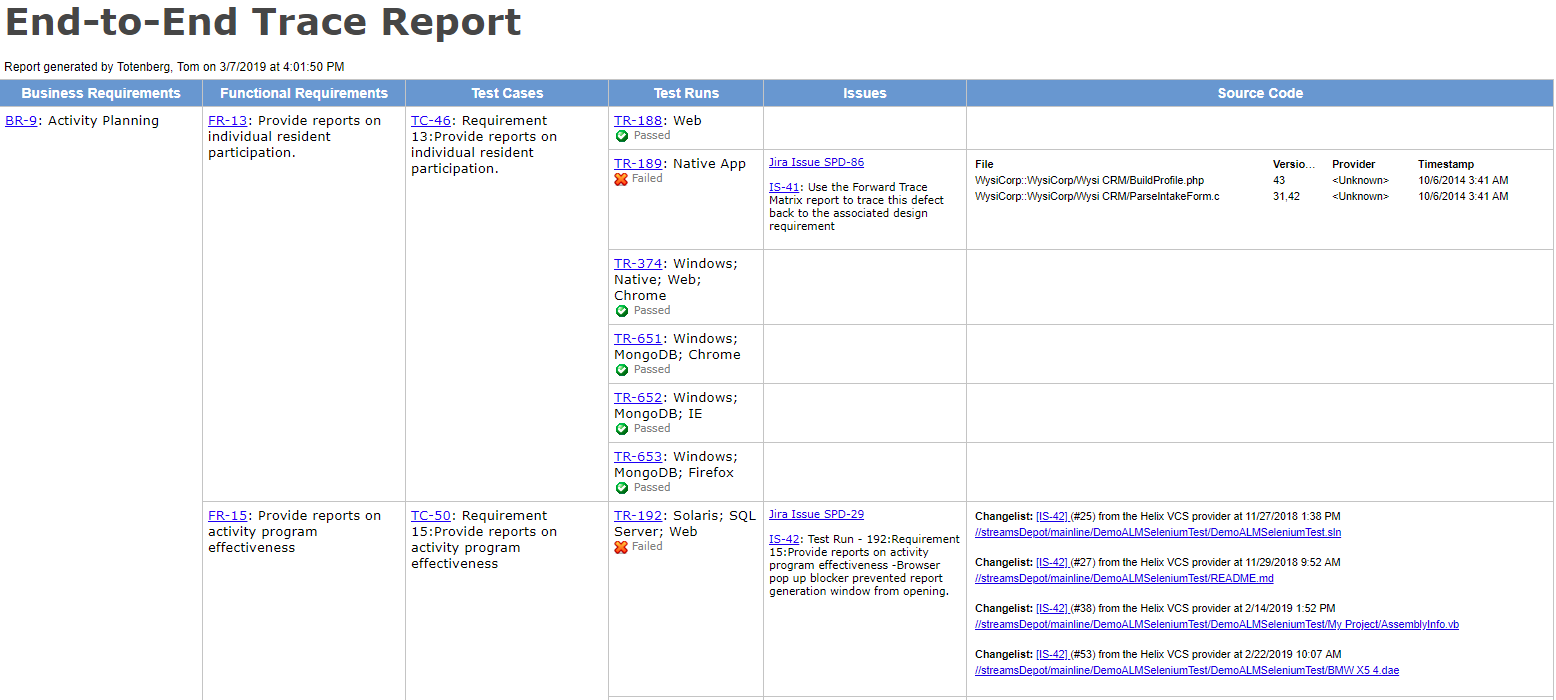
A medical device testing strategy must incorporate compliance processes and technical testing strategies for better performance and effectiveness of medical devices. Vectorcast by vector is the solution for fda compliant and iec 62304 compliant medical embedded application testing. Software testing checks that a given program correctly implements requirements to the iec 62304 standard. General validation principles of medical device software or the validation of software used to design develop or manufacture medical devices.
We have a unique understanding of the complexity of medical devices due to the criticality and integrated ecosystem of hardware and software. With matlab and simulink you can. Manufacturers need to have a strong testing strategy in place right from the design stage as performing an exhaustive testing of a produced device is ineffective and inefficient. Manufacturers that have embedded software in medical devices need to follow robust software development and testing processes to capture all information necessary for compliance.
Such processes ensure effective performance accurate reading and safety of devices. Software testing is a critical part of ensuring the performance of medical devices to pass fda audits and support the guidelines defined in iec 62304. And that calls for medical device development tools. Simulate and test embedded software alongside mechatronic systems early.
Medical devices used in patient care directly impacts human lives. Medical device testing needs to be thorough. Iec 62304 medical device software software life cycle processes specifies life cycle requirements for the development to medical software and software within medical devices. Our quality solutions have supported dozens of leading brands in fact medical devices make up our largest us sector.


%20Design%20Verification%20%26%20Validation.png?width=1700&name=(cover)%20Design%20Verification%20%26%20Validation.png)













